Compound Semiconductor
What is a Semiconductor?
Semiconductor is the generic term for materials that have electrical conductivity between conductors (like copper and aluminum), and insulators (like rubber and glass). Of the 92 elements, only a few can be used as semiconductor materials. Silicon, germanium, and selenium are examples of materials that are semiconductors. Among them, silicon has been and still is the most common semiconductor. It has widespread commercial applications and is readily available.
What is a Compound Semiconductor?
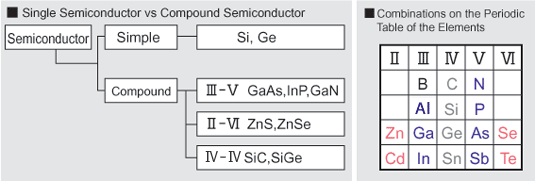
Compound semiconductors are semiconductors that are made from two or more elements. Silicon is made from a single element, and therefore is not a compound semiconductor.
Most compound semiconductors are from combinations of elements from GroupIII and GroupV of the Periodic Table of the Elements (GaAs, GaP, InP and others). Other compound semiconductors are made from Groups II and VI (CdTe, ZnSe and others). It is also possible to use different elements from within the same group (IV), to make compound semiconductors such as SiC.
In the past, compound semiconductors were not used in the widespread commercial applications and high production volumes typical for silicon. These crystals are more difficult to grow than silicon. The number of defects in the crystal is higher, and the cost of making the crystal is higher. Compound semiconductors also tend to be more fragile. All of these factors limited the growth of compound semiconductors for commercial use.
In recent years, however, the cost of manufacturing compound semiconductors has come down. It is still much higher than silicon, but at the same time, the special properties of these crystals have become more important for certain applications. Because of their fundamental material properties, compound semiconductors can do things that simply aren't possible with silicon.